Introduction
 On November 4, 2024, the FDA approved the first and only treatment for Dry Age-Related Macular Degeneration (AMD) in the U.S., the Valeda® Light Delivery System.
On November 4, 2024, the FDA approved the first and only treatment for Dry Age-Related Macular Degeneration (AMD) in the U.S., the Valeda® Light Delivery System.
The Valeda® treatment was approved in Europe in 2019. Since then, more than 500,000 treatments have been performed with much success and no significant side effects.
The treatment is non-invasive, painless and uses several wavelengths of light to improve the health of the macula.
FDA clinical studies in the U.S. demonstrated that this non-invasive light therapy improved vision in most AMD study patients.
Our Experience
On December 2, 2024, Caplan Eye Clinic became the 1st eye center in the United States to receive delivery of the FDA approved Valeda® Light Delivery System.
On December 5, 2024, Dr. Chad Caplan became the 1st physician in the United States to perform Valeda light therapy on a patient following FDA approval. Dr. Daniel Caplan & Dr. Brendon Sumich were the 2nd and 3rd physicians respectively in the U.S. to perform Valeda light therapy on a patient.
Caplan Eye Clinic, with Dr. Daniel Caplan as the Principal Investigator, was the only eye center in Louisiana and one of only 11 eye centers in the U.S. to participate in an FDA Clinical Trial of the Valeda® Light Delivery System (2023-2024).
Background
 AMD (Age-related Macular Degeneration) is a progressive disease of the retina (the part of the eye that detects visual signals and sends them to the brain). AMD is the leading cause of vision loss in people over 65 years. There are two categories of AMD: the Dry form that accounts for 80 to 90% of all AMS cases and the Wet form that accounts for 10 to 20%.
AMD (Age-related Macular Degeneration) is a progressive disease of the retina (the part of the eye that detects visual signals and sends them to the brain). AMD is the leading cause of vision loss in people over 65 years. There are two categories of AMD: the Dry form that accounts for 80 to 90% of all AMS cases and the Wet form that accounts for 10 to 20%.
The Valeda treatment is for Dry AMD.
The exact cause of AMD is not known but there is evidence to suggest that several factors are involved. These include reduced blood flow to the eye through the small blood vessels and buildup of waste products in the cell.
There is currently no cure for AMD. However, LumiThera has developed a device called the Valeda® Light Delivery System to treat Dry AMD. The device has been approved for the treatment of Dry AMD in Europe since 2019. It was approved by the U.S. Food & Drug Administration (FDA) on November 4, 2024 for use in the U.S. for the treatment of Dry AMD. The Valeda® Light Delivery System provides a light-based therapy called Photobiomodulation (PBM) to the retinal tissue.
PBM is the application of specific wavelengths of light to the eye. The Valeda® Light Delivery System uses Light Emitting Diodes (LED) which are very low powered and do not cause any heat damage to the eye. This is NOT a laser device.
The FDA has approved similar wavelengths of light for use in other clinical devices for medical conditions such as wrinkles around the eyes, temporary relief of minor muscle and joint pain, arthritis and muscle spasm, and to temporarily improve blood flow in areas where it is applied.
Treatment
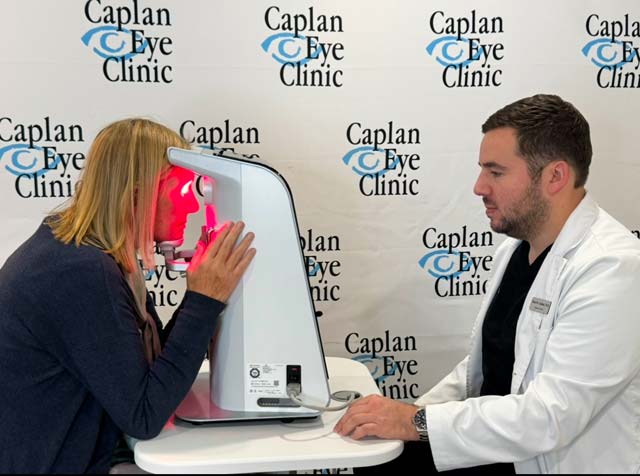 This treatment plan includes 3 series of 9 treatments each over a 1-year period (27 treatments total). The 9 treatments are performed approximately 3 times a week over a 3 to 4-week period of time. Each treatment session takes about 10 minutes.
This treatment plan includes 3 series of 9 treatments each over a 1-year period (27 treatments total). The 9 treatments are performed approximately 3 times a week over a 3 to 4-week period of time. Each treatment session takes about 10 minutes.
There is no special preparation needed prior to the treatment, and your eyes will not be dilated. You will need to remove your glasses or contact lenses. No drugs or needles are used in the treatments. Your eye(s) that are eligible to be in the treatment plan will receive the PBM treatment using the Valeda® Light Delivery System, which uses 3 different wavelengths: red light, near-infrared (invisible light) and yellow light. The instrument looks similar to the devices you look into to test your vision. Your eye(s) will be given a treatment of different light waves through both open and closed eyelids to stimulate the back of the eye.
Treatment Visits
 You will receive a total of nine treatments over a period of three to four (3 to 4) weeks during each series of treatments.
You will receive a total of nine treatments over a period of three to four (3 to 4) weeks during each series of treatments.
Each treatment takes approximately five (5) minutes for each eye being treated. During the treatment, you will be told when to open your eyes and when to close them. There are no eye drops used at the treatment visit. There is no discomfort during the treatment.
First re-treatment visit of each Series of treatments – months 4 and 8: You will receive a detailed eye exam and usually the tests that were performed initially. Then the treatment will be performed.
Potential Risks
The Valeda Light Delivery System has been in use in Europe since 2019. Among the more than 500,000 treatments performed in Europe, there have been no known significant adverse effects reported.
Limitations of Valeda® PBM Therapy AMD is a progressive disease that may lead to vision loss over time. While the European Valeda® experience since 2019 and the American FDA studies have shown promising results, there is no guarantee that your vision will improve following Valeda® treatment. Even with Valeda® treatment, your dry macular degeneration could still progress to wet AMD and loss of vision.
More Information
If you have early to intermediate dry macular degeneration and wish to learn more about the Valeda Light Delivery System, call 504-888-2600 for an appointment, or send email to: info@caplaneye.com.
For our out-of-town visitors, here are our nearby hotels and attractions for you your reference.